In the following reaction,
Zn (s) + CuSO4 (aq) → ZnSO4 (aq) + Cu (s) :
A) Zn is the reducing agent and CuSO4 is the oxidizing agent.
B) CuSO4 is the reducing agent and Zn is the oxidizing agent.
C) ZnSO4 is the reducing agent and Cu is the oxidizing agent.
D) 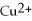
Is the reducing agent and Zn2+ is the oxidizing agent.
E) none of the above
Correct Answer:
Verified
Q44: The oxidizing agent typically:
A)loses electrons.
B)gains oxygen.
C)is the
Q45: Oxidation typically involves:
A)the loss of electrons.
B)the loss
Q48: The rusting of iron is an example
Q49: Identify the substance being oxidized in the
Q52: In the following reaction,
Mg (s)+ 
Q52: Which of the following are typically TRUE
Q53: Identify the reducing agent in the following
Q57: Reduction typically involves:
A)the loss of electrons.
B)the gain
Q59: Reduction involves which of the following?
1.Loss
Q60: Oxidation involves which of the following?
1.Loss
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents