How many protons, neutrons, and electrons are in the following isotope, respectively? 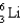
A) 3, 3, 3
B) 3, 6, 3
C) 6, 3, 6
D) 6, 3, 3
E) 3, 6, 6
Correct Answer:
Verified
Q84: The element that corresponds to the following
Q85: Nitrogen has five core (innermost) electrons.
Q86: In a neutral atom, there is an
Q87: Fluorine is classified as a halogen.
Q88: Consider the table given below. 
Q90: The elements that are good conductors of
Q91: The symbol and atomic number of the
Q92: The Lewis dot symbol shown below could
Q93: Ne is classified as a noble gas.
Q94: In the following set, which atom is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents