Consider the following energy profile for a reaction. 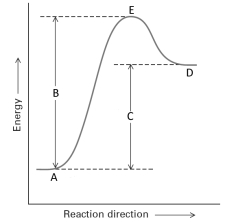 Answer with the following questions with the letters shown in the profile.
Answer with the following questions with the letters shown in the profile.
-The reactants are represented by the letter _______.
Correct Answer:
Verified
Q40: Which of the following samples has the
Q41: Consider the following two statements.
"Energy can be
Q42: Consider the following reaction. Q43: Consider the following energy profile for a Q44: An indicator, HIn, shows a color change Q46: Addition of a catalyst to a reaction Q47: In the following reaction, the products have Q48: The molar mass of sulfur trioxide is Q49: The following energy profile represents an Q50: Consider the following energy profile for a
PCl5(g) ![]()
exergonic reaction.
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents