The equilibrium constant for the reaction of bromine with chlorine to form bromine monochloride is 58.0 at a certain temperature. Br2(g) + Cl2(g)  2BrCl(g)
2BrCl(g)
What is the equilibrium constant for the following reaction?
BrCl(g) 
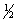 Br2(g) +
Br2(g) + 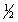 Cl2(g)
Cl2(g)
A) 2.97 × 10-4
B) 1.72 × 10-2
C) 3.45 × 10-2
D) 1.31 × 10-1
E) > 1.00
Correct Answer:
Verified
Q24: Consider the reactions of cadmium with the
Q25: H2SO3(aq) Q26: The equilibrium constant, Kp , for the Q27: About half of the sodium carbonate produced Q28: In water, the following equilibrium exists: H+(aq) Q30: Nitrogen dioxide decomposes according to the reaction Q31: Write the mass-action expression, Qc , for Q32: The equilibrium constant, Kp, has a value Q33: Consider the following two equilibria and their Q34: Hydrogen sulfide will react with water as![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents