Nitric oxide and bromine were allowed to react in a sealed container. When equilibrium was reached PNO = 0.526 atm, 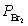 = 1.59 atm, and PNOBr = 7.68 atm. Calculate Kp for the reaction. 2NO(g) + Br2(g)
= 1.59 atm, and PNOBr = 7.68 atm. Calculate Kp for the reaction. 2NO(g) + Br2(g)  2NOBr(g)
2NOBr(g)
A) 7.45 × 10-3
B) 0.109
C) 9.18
D) 91.8
E) 134
Correct Answer:
Verified
Q49: The reaction system POCl3(g) Q50: At 850°C, the equilibrium constant Kp for Q51: Nitric oxide is formed in automobile exhaust Q52: At high temperatures, carbon reacts with O2 Q53: The equilibrium constant, Kp , for the Q57: A mixture of 0.600 mol of bromine Q58: The equilibrium constant Kc for the reaction Q59: At 25°C, the equilibrium constant Kc for Q63: SO2 reacts with O2 to produce SO3. Q65: Hydrogen iodide, HI, is formed in an![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents