Hydrogen sulfide can be formed in the following reaction: H2(g) + 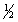 S2(g)
S2(g)  H2S(g) H°rxn = -92 kJ
H2S(g) H°rxn = -92 kJ
The equilibrium constant Kp = 106 at 1023 K. Estimate the value of Kp at 1218 K.
A) 5.05
B) 18.8
C) 34.7
D) 88.9
E) 598
Correct Answer:
Verified
Q67: The reaction system CS2(g) + 4H2(g)
Q68: The following reaction is at equilibrium at
Q69: The following reaction is at equilibrium in
Q70: Methanol can be synthesized by combining
Q71: At 450°C, tert-butyl alcohol decomposes into water
Q73: Sodium hydrogen carbonate decomposes above 110°C to
Q74: Magnesium carbonate dissociates to magnesium oxide and
Q75: The reaction of nitric oxide to form
Q76: Hydrogen bromide will dissociate into hydrogen
Q77: The following reaction is at equilibrium
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents