Determine the likely structure for a compound A (C6H10O) ,which is found to decolorize bromine in carbon tetrachloride.Its spectral data is as follows: 1H NMR IR
Triplet, 1.0 singlet, 2.4 2200 cm-1 (sharp)
Singlet, 1.4 singlet, 3.4 3300 cm-1 (sharp)
Quartet, 1.6 3500 cm-1 (broad) 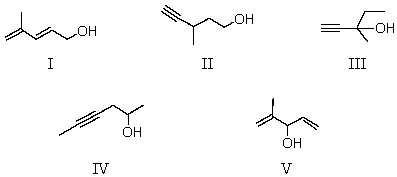
A) I
B) II
C) III
D) IV
E) V
Correct Answer:
Verified
Q60: What is the structure of the compound
Q61: In NMR terminology,protons Ha and Hb are
Q62: Q63: How many 13C signals would you expect Q64: How will the methyl carbon appear in Q66: How many 13C signals would 1,4-dimethylbenzene give? Q67: A prominent (M 1+ Q68: How many 13C signals would 1,3-dichlorobenzene give? Q69: The broadband proton-decoupled 13C NMR spectrum of Q70: How many signals will be recorded in![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents