The heat of vaporization for water is 540 cal/g.How many kilocalories are needed to change 22 g of liquid water to steam at 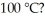
A) 540 kcal
B) 12 kcal
C) 12000 kcal
D) 25 kcal
E) 1.8 kcal
Correct Answer:
Verified
Q47: The heat of fusion for water is
Q48: If the heat of fusion for water
Q49: A burn from steam at 100 °C
Q50: A kilocalorie of heat is required to
Q51: When a solid is converted directly to
Q53: The formation of a gas resulting from
Q54: The physical state(s)present when a substance is
Q55: How many calories are required to raise
Q56: The specific heat of a substance is
Q57: Raising the temperature of 10.0 g of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents