Which of the following best represents the tetrahedral shape of sp3 hybridized carbons? (Note: a solid wedge is pointed towards you and a dashed wedge is pointed away.)
A) 
B) 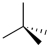
C) 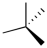
D) 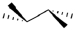
Correct Answer:
Verified
Q43: What is the most common type of
Q49: cis -1,2-Dimethylcyclohexane and trans -1,2-dimethylcyclohexane are examples
Q55: Three physical properties shown below beside different
Q56: Give the IUPAC name for the following
Q59: The following two structures would be classified
Q61: Functional groups are to organic chemistry as
Q64: An unlimited number of carbon atoms can
Q75: Most organic compounds contain ionic bonds.
Q76: Carbon atom tends to form tetrahedral structures
Q77: Isomers differ from each other in the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents