Suppose the reaction 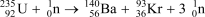 produces 1.664
produces 1.664  1010 kJ/mol of energy.Calculate the change in mass in grams that occurs when one mole of U-235 reacts with one mole of neutrons.E = mc2,where c = 2.998
1010 kJ/mol of energy.Calculate the change in mass in grams that occurs when one mole of U-235 reacts with one mole of neutrons.E = mc2,where c = 2.998  108 m/s; 1 kg = 6.0221415
108 m/s; 1 kg = 6.0221415  1026 amu; 1 J = 1kg . m2/s2.
1026 amu; 1 J = 1kg . m2/s2.
A) 0.185 g
B) 0.555 g
C) 0.898 g
D) 5.41 g
E) 9.22  10-17 g
10-17 g
Correct Answer:
Verified
Q23: When carbon burns to produce carbon
Q24: The isotope "belt of stability" is an
Q25: What quantity of energy would be
Q26: The peak in nuclear binding energy/nucleon occurs
Q29: When bombarded by a proton,a lithium-7
Q30: What quantity of energy would be produced
Q31: Which of the following statements regarding the
Q32: What quantity of energy would be
Q33: When 4 protons and 4 neutrons combine
Q134: Compare the energy released by the collisions
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents