Permanganate ions can oxidize sulfite in basic solution according to the following equation.The relevant standard reduction potentials are 0.59 V for the manganese half-reaction and -0.92 V for the sulfur half-reaction.Determine the cell potential for the reaction at 298 K with the concentrations in the table.
2 MnO4-(aq)+ 3 SO32-(aq)+ H2O( 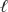 ) 2 MnO2(s)+ 3 SO42-(aq)+ 2 OH-(aq)
) 2 MnO2(s)+ 3 SO42-(aq)+ 2 OH-(aq)
Correct Answer:
Verified
Q151: What is the most important use for
Q161: How does a fuel cell differ from
Q162: Starting with
Q163: The capacity of a battery usually
Q165: Estimate the value of Kf for
Q166: Because of recent advances in recovery technology,the
Q169: If,in using a lead-acid battery to start
Q170: A concentration cell is made using Ni2+
Q171: Suppose a NiMH battery is rated
Q172: Chromium often is electroplated on other metals
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents