Given the following data,calculate the approximate value of the equilibrium constant at 298 K for the reaction,Ni(NH3) 62+(aq) + 3 en(aq) Ni(en) 32+(aq) + 6 NH3(aq) ,where en represents ethylenediamine.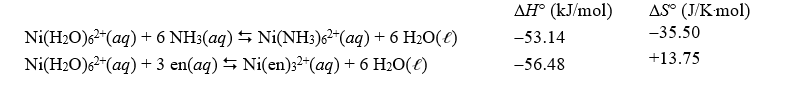
A) 1.90 10-2
B) 52.7
C) 3.77
D) 1.44 103
E) 6.94 10-4
Correct Answer:
Verified
Q22: Which is the correct formula for potassium
Q23: The correct formula for ammonium tetracyanoplatinate(II) is
Q25: EDTA is an example of a _
Q30: What is the correct formula for sodium
Q36: Ethylenediamine is an example of a _
Q40: How many chelation sites and donor groups
Q50: The structure of the acetylacetonate ligand is
Q54: The reaction,Cr(NH3)63+(aq)+ 3 en(aq)
Q57: The interaction of a metal ion with
Q71: Which of the following is a chelation
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents