Phosphoric acid is a triprotic acid in water,ionizing in the following sequential steps: H3PO4 + H2O H2PO4- + H3O+ 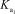 H2PO4- + H2O HPO42- + H3O+
H2PO4- + H2O HPO42- + H3O+ 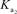 HPO42- + H2O PO43- + H3O+
HPO42- + H2O PO43- + H3O+
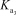 What is the appropriate Kb expression for a sodium phosphate solution?
What is the appropriate Kb expression for a sodium phosphate solution?
A) 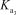
B) 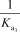
C) 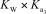
D) 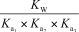
E) 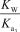
Correct Answer:
Verified
Q41: What is the actual concentration of molecular
Q79: The degree of ionization _
A)increases with increasing
Q82: Phosphoric acid is a triprotic acid
Q83: What is the percent dissociation of
Q84: What is the concentration of [OH-]
Q85: What is the pH of a
Q86: What is the percent dissociation of
Q89: What is the actual concentration of
Q90: Phosphoric acid is a triprotic acid
Q91: What is the pH of a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents