Phosphoric acid is a triprotic acid in water,ionizing in the following sequential steps: H3PO4 + H2O H2PO4- + H3O+ 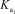 = 7.11 *10-3
= 7.11 *10-3
H2PO4- + H2O HPO42- + H3O+
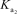 = 6.32 *10-8
= 6.32 *10-8
HPO42- + H2O PO43- + H3O+
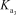 = 4.5 *10-13
= 4.5 *10-13
What is the pH of a 0.20 M Na3PO4 solution?
A) 1.66
B) 5.82
C) 10.50
D) 12.75
E) 12.82
Correct Answer:
Verified
Q79: The degree of ionization _
A)increases with increasing
Q90: Phosphoric acid is a triprotic acid
Q91: What is the pH of a
Q93: When values of Ka are small
Q96: Sulfuric acid is a diprotic acid.What is
Q97: What is the hydronium ion concentration
Q98: What is the percent dissociation of
Q99: Phosphoric acid is a triprotic acid
Q100: What is the pH of a
Q111: The analysis label on a 500 mL
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents