A 0.500 g sample of an unknown substance was titrated with a 0.1 M HCl solution.Another 0.500 g sample of it was titrated with a 0.1 M NaOH solution.The resulting titration curves are illustrated here.Given the following possibilities,what is the sample? 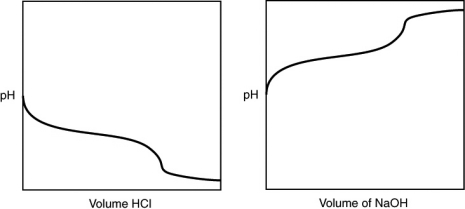
A) Na2CO3
B) NaHCO3
C) H2CO3
D) CO2
E) There is no way to tell.
Correct Answer:
Verified
Q21: At the equivalence point of a strong
Q22: Acid-base indicators need to have very intense
Q28: Bromocresol green is yellow in its acidic
Q72: When an acetic acid solution is titrated
Q110: Acid-base indicators change color _
A) exactly when
Q149: To simulate the pH of blood,which
Q151: What is true about the pKa of
Q152: What is true about the pKa of
Q156: The following titration curve is most likely
Q158: In which of the following titrations would
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents