Gaseous carbon monoxide and hydrogen gas can be used to prepare methanol (CH3OH).Under equilibrium conditions at 427 C,the partial pressures of the components are as follows: 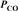 = 1.44 atm
= 1.44 atm 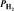 = 4.25 atm
= 4.25 atm 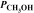 = 2.30 atm
= 2.30 atm
What is the value of the equilibrium constant for the reaction in which one mole of methanol is produced?
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q84: Chemical equilibrium arises from the _ of
Q96: A qualitative interpretation of the effect
Q126: When plotting ln K vs.1/T,a linear
Q127: For the equilibrium 2 OF2(g)
Q129: Given the following two measurements of
Q130: Consider the equilibrium CO(g)+ 2 H2(g)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents