Assuming that each of the following graphs has the same concentration and time axes,which has the greatest initial rate of disappearance of reactant?
A) 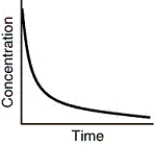
B) 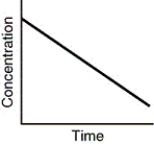
C) 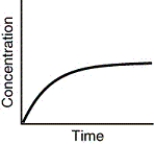
D) all are equivalent at the start of the reaction
Correct Answer:
Verified
Q30: For the reaction 4 NH3(g)+ 7
Q31: For the reaction 3 I-(aq)+ H3AsO4(aq)+
Q33: If the average rate of reaction
Q33: Which of the following statements is
Q34: The rate of disappearance of HI
Q36: Which of the following statements regarding the
Q37: Which of the following statements regarding the
Q38: Which of the following is not a
Q39: For the reaction 3 I-(aq)+ H3AsO4(aq)+
Q40: The following graph shows the kinetics
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents