Given the following reactions,what is the overall enthalpy change for the following reaction?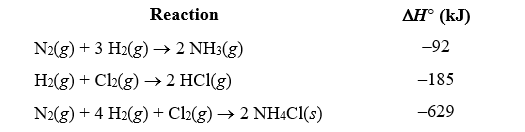
A) -38 kJ
B) -176 kJ
C) -352 kJ
D) -445 kJ
E) -454 kJ
Correct Answer:
Verified
Q92: Which statement regarding combustion of a sample
Q106: In an experiment,110.0 g of iron is
Q107: Use the following information to determine
Q108: Nitroglycerin decomposes to form carbon dioxide,water
Q109: When 7.29 g hydrochloric acid (36.46 g/mol)and
Q110: The freezing point of ammonia (NH3,17.04 g/mol)is
Q113: When a 13.0 g sample of NaOH(s)dissolves
Q114: Isooctane is a good model for gasoline.When
Q115: In an experiment,100.0 g of water at
Q116: When 1.14 g of octane (molar mass
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents