Ammonium nitrate (80.04 g/mol)decomposes at high temperatures to form nitrogen,oxygen,and water vapor.Suppose 750 grams decomposes at 350°C and 0.98 atm,generating gases that expand by about 960 L.How much P-V work is done? What is the approximate change in internal energy ( E )of the reaction system? (1 L . atm = 101.3 J)
NH4NO3(s) N2(g)+ 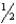 O2(g)+ H2O(g) H =-118 kJ/mol
O2(g)+ H2O(g) H =-118 kJ/mol
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q98: Describe the difference between the change
Q108: Explain what is meant by the term
Q120: If steam expands in the cylinder of
Q167: A system is compressed from 40.0 L
Q168: Suppose 250 g of water at 95°C
Q169: A sample of propane gas is contained
Q171: The molar heat capacities of zinc,titanium,and iron
Q173: In an experiment,36.0 grams of ice
Q175: When one mole of potassium hydroxide dissolves
Q177: Label the following as exothermic or endothermic.
(a)water
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents