Tetracyanoethylene has the skeleton shown below: 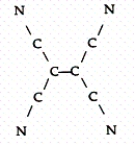 From its Lewis structure determine the following:
From its Lewis structure determine the following:
-How many sigma and pi bonds are in the molecule?
A) 4 sigma and 5 pi
B) 6 sigma and 8 pi
C) 9 sigma and 8 pi
D) 9 sigma and 9 pi
E) 5 sigma and 8 pi
Correct Answer:
Verified
Q17: The hybridization of the central atom,Al,in AlBr3
Q18: The hybridization of the central atom in
Q19: What hybridization is predicted for the nitrogen
Q20: In the molecule C2H4 the valence orbitals
Q21: The hybridization of Se in SeF6 is
A)sp
B)sp2
C)sp3
D)dsp3
E)d2sp3
Q23: Consider the molecule C2H4.The hybridization of each
Q24: Which of the following substances contains two
Q25: Tetracyanoethylene has the skeleton shown below:
Q26: Consider the skeletal structure shown below:
N-C-C-N
Draw the
Q27: Tetracyanoethylene has the skeleton shown below:
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents