If a constant current of 5.0 amperes is passed through a cell containing Cr3+ for 1.0 hour,how many grams of Cr will plate out onto the cathode? (The atomic mass of Cr is 51.996. )
A) 9.7 g
B) 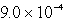 g
g
C) 3.2 g
D) 29 g
E) 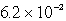 g
g
Correct Answer:
Verified
Q122: Which of the following are incorrectly paired?
A)Alumina
Q123: Balance the following equation: Cr2O72- +
Q124: If an electrolysis plant operates its
Q125: Balance the following equation: MnO4- +
Q126: What mass of Ti(s)may be deposited
Q128: An unknown metal (M)is electrolyzed.It took 74.1
Q129: A solution of MnO42- is electrolytically reduced
Q130: Consider a galvanic cell with a
Q131: Nickel is electroplated from a NiSO4 solution.A
Q132: Gold (atomic mass = 197.0)is plated from
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents