The solubility of CaSO4 in pure water at 0oC is 1.14 gram(s) per liter.The value of the solubility product is
A) 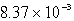
B) 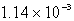
C) 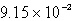
D) 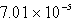
E) none of these
Correct Answer:
Verified
Q11: Calculate the concentration of the silver
Q12: The solubility of an unknown salt,M3Z2, at
Q13: The solubility of an unknown salt,MZ3, at
Q14: The solubility in mol/L of Ag2CrO4
Q15: It is observed that 7.50 mmol of
Q17: An unknown salt,M3Z,has a Ksp of
Q18: The concentration of OH- in a
Q19: Barium carbonate has a measured solubility
Q20: Find the solubility (in mol/L)of lead(II)chloride,PbCl2,at
Q21: Calculate the solubility of Ag2CrO4 (Ksp
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents