Multiple Choice
Consider the reaction: 2SO2(g) + O2(g)  2SO3(g) at constant temperature.Initially a container is filled with pure SO3(g) at a pressure of 2 atm,after which equilibrium is reached.If y is the partial pressure of O2 at equilibrium,the value of Kp is:
2SO3(g) at constant temperature.Initially a container is filled with pure SO3(g) at a pressure of 2 atm,after which equilibrium is reached.If y is the partial pressure of O2 at equilibrium,the value of Kp is:
A) 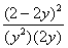
B) 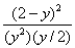
C) 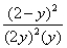
D) 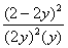
E) none of these
Correct Answer:
Verified
Related Questions
Q23: Consider the reaction: CaCl2(s)+ 2H2O(g) 
Q24: Consider the following reaction: CS2(g)+ 4H2(g)
Q25: For the reaction given below,2.00 moles of

