Consider the following equilibrium: 2NOCl(g)  2NO(g) + Cl2(g) with K = 1.6 10-5.In an experiment,1.00 mole of pure NOCl and 1.00 mole of pure Cl2 are placed in a 1.00-L container.
2NO(g) + Cl2(g) with K = 1.6 10-5.In an experiment,1.00 mole of pure NOCl and 1.00 mole of pure Cl2 are placed in a 1.00-L container.
-If x moles of NOCl react,what is the equilibrium concentration of Cl2?
A) x
B) 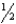 x
x
C) 1 + x
D) 1 + 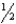 x
x
E) 1 + 2x
Correct Answer:
Verified
Q66: At a certain temperature K for the
Q67: Consider the following equilibrium: 2NOCl(g)
Q68: Ammonia is prepared industrially by the
Q69: At 500.0 K,one mole of gaseous
Q70: For the reaction below,Kp = 1.16 at
Q72: Consider the following equilibrium: 2NOCl(g)
Q73: Consider the following equilibrium: 2H2(g)+ X2(g)
Q74: Consider the following equilibrium: 2H2(g)+ X2(g)
Q75: Exactly 1.0 mol N2O4 is placed in
Q76: The following questions refer to the equilibrium
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents