A sample consisting of CO2(g) and CO2(s) at equilibrium at -78°C and 1 atm pressure is heated to -30°C and the pressure is increased to 8 atm.Based on the phase diagram below,what will happen? 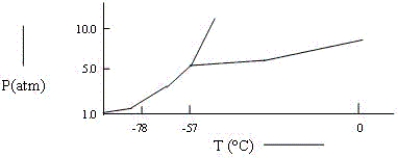
A) At equilibrium,only CO2(g) will be present.
B) All of the CO2 will be converted to CO2(l) .
C) At equilibrium,CO2(g) and CO2(l) will be present.
D) The melting point of the CO2(s) will decrease.
E) None of these.
Correct Answer:
Verified
Q110: Shown below is a phase diagram for
Q111: Below is a phase diagram for compound
Q112: The particularly strong dipole-dipole interaction between hydrogen
Q113: The triple point of iodine is at
Q114: A certain substance has the phase diagram
Q116: The relatively weak forces that exist among
Q117: If you have 10.0 moles of BH3
Q118: The heat of combustion of bituminous
Q119: Which statement regarding water is true?
A)Energy must
Q120: Based on the phase diagram shown
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents