Multiple Choice
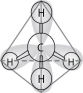 Figure 2.8
Figure 2.8
-In the methane molecule shown in Figure 2.8, bonds have formed that include both the s orbital valence electrons of the hydrogen atoms and the p orbital valence electrons of the carbon. The electron orbitals in these bonds are said to be
A) double orbitals.
B) tetrahedral orbitals.
C) complex orbitals.
D) hybrid orbitals.
E) polar orbitals.
Correct Answer:
Verified
Related Questions
Q24: You have two beakers. One contains pure
Q70: Many mammals control their body temperature by

