Calculate the value of K for the following reaction at 298 K. 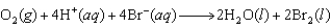 given the following reduction potentials
given the following reduction potentials 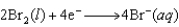 E = +1.077 V
E = +1.077 V 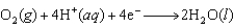 E = +1.229 V
E = +1.229 V
A) 1.92 1010
B) 3.70 102
C) 1.04
D) 1.18
E) 3.54 101
Correct Answer:
Verified
Q98: Cadmium is a toxic heavy metal.
Q99: A concentration cell is constructed by using
Q100: An electrochemical cell is constructed with
Q101: The unit of electrical power, watt (W),
Q102: The average electrical current delivered if
Q104: The Energizer Bunny in the television commercial
Q105: A typical D battery has a
Q106: The capacity of a battery usually
Q107: The charge supplied by a battery can
Q108: Which one of the following is not
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents