Potassium superoxide reacts violently with water to produce potassium hydroxide and oxygen according to the following balanced equation. What would the temperature be if 5.0 g of KO2 reacts to produce a pressure of 1.0 atm and 2.0 L of O2 gas?
4KO2(s) 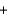 2H2O(l)
2H2O(l)  4KOH(s)
4KOH(s) 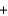 3O2(g)
3O2(g)
A) 150 C
B) 30 C
C) - 12 C
D) 190 C
E) 74 C
Correct Answer:
Verified
Q64: When a certain acid and a
Q65: Nitrous oxide (N2O, also known as
Q66: What is the molar mass of
Q67: What is the density of propane
Q68: When a certain acid and a
Q70: The density of a pure gas at
Q71: Indicate the gas with the greatest density
Q72: What is the pressure of NO
Q73: Which of the following samples of an
Q74: Which one of the following gases could
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents