Using the Following Data, Determine the Standard Cell Potential Zn2+(aq) + Pb(s)
Half-Reaction Standard Reduction Potential
Zn2+(aq) + 2e-
Using the following data, determine the standard cell potential 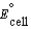 for the electrochemical cell constructed using the following reaction, where zinc is the anode and lead is the cathode. Zn(s) + Pb2+(aq) Zn2+(aq) + Pb(s)
for the electrochemical cell constructed using the following reaction, where zinc is the anode and lead is the cathode. Zn(s) + Pb2+(aq) Zn2+(aq) + Pb(s)
Half-reaction Standard Reduction Potential
Zn2+(aq) + 2e- Zn(s) -0.763
Pb2+(aq) + 2e- Pb(s) -0.126
A) +0.637 V
B) -0.637 V
C) +1.274 V
D) -0.889 V
E) +0.889 V
Correct Answer:
Verified
Q4: This is a true story; can
Q4: Oxidation refers to _
A)an increase in oxidation
Q5: This is a true story; can you
Q6: Which one of the following items does
Q9: Reduction refers to _
A)a decrease in oxidation
Q12: This is a true story; can
Q20: Reduction is the _
A)gain of electrons.
B)loss of
Q32: Proteins containing a certain functional group
Q37: Which statement about a cathode in a
Q76: The standard hydrogen electrode is _
A)used to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents