For the electrochemical cell indicated below where zinc is being oxidized, identify the components labeled as a-d. Use the terms zinc nitrate solution, copper nitrate solution, copper electrode, and salt bridge. Also, identify the cathode and the anode, and label the electrodes as + and -. 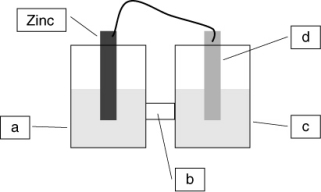
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q30: Which statement regarding voltaic cells is not
Q87: An electrochemical cell at 298 K
Q93: Identify the strongest reducing agent in
Q96: Copper is oxidized by nitric acid.
Q134: What must be true about the
Q135: Starting with
Q147: What is the change in free energy
Q148: What constitutes a standard hydrogen electrode?
Q159: How much work can be done using
Q166: If in using a lead-acid battery to
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents