Using the thermodynamic data below, determine the equilibrium constant for the conversion of oxygen to ozone at 2000 K. Substance H  (kJ/mol) G
(kJ/mol) G  (kJ/mol) S (J/mol · K)
(kJ/mol) S (J/mol · K)
O2(g) 0 0 205.0
O3(g) 142.3 163.4 237.6
3O2(g) 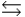 2O3(g)
2O3(g)
A) 2.04 * 107
B) 5.44 *10-14
C) 2.67 * 10-8
D) 2.91 * 10-9
E) 3.65 *10-12
Correct Answer:
Verified
Q75: A perturbation or stress to a chemical
Q77: What is the value of the
Q78: As T approaches infinity, lnK approaches _
A)
Q79: Several groups of general chemistry lab
Q83: For a chemical reaction at equilibrium, which
Q84: Chemical equilibrium arises from the _ of
Q85: For a particular hypothetical reaction, 2A
Q85: A sealed tube containing an equilibrium
Q103: Students often write expressions for the equilibrium
Q108: How and why are the expressions for
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents