In the sodium chloride unit cell, the chloride ions form a cube in which each side is arranged like the following figure. The circles represent the positions of the chloride ions on one square face of the cube. All the other faces are the same. What is the name of this unit cell? 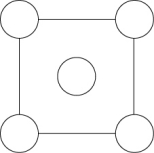
A) cubic
B) chloride-centered cubic
C) face-centered cubic
D) x-face cubic
E) body-centered cubic
Correct Answer:
Verified
Q20: Pure solid metals _
A)do not crystallize.
B)are amorphous.
C)often
Q21: Aluminum (Al) crystallizes as a face-centered unit
Q28: Gold has a face-centered cubic structure with
Q29: Which is not true about a crystallographic
Q30: Polonium crystallizes in a simple cubic pattern.
Q38: How many nearest neighbor atoms are there
Q39: How many nearest neighbor atoms are there
Q40: Which unit cell contains the most atoms?
A)fcc
B)bcc
C)cubic
D)both
Q47: Iron (Fe) has a density of 7.874
Q54: The alpha form of polonium (Po) crystallizes
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents