The XRD scan of the face-centered cubic (fcc) structure of sodium chloride showed that there was a distance of 562.8 pm between "layers of ions." Given that the fcc unit cell has a volume of 178.26 *10-24 cm3, to which distance does the 562.8 pm correspond?
A) 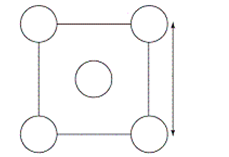
B) 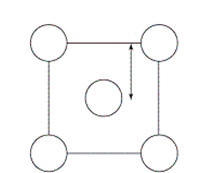
C) 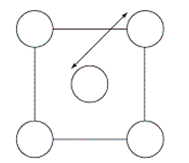
D) 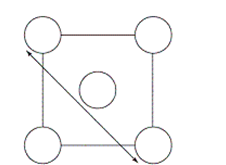
Correct Answer:
Verified
Q101: Aluminum (Al) has a density of 2.70
Q104: If a body-centered cubic unit cell
Q108: Graphite and diamond are examples of _
Q111: Just as visible light is diffracted by
Q115: Yttrium-barium-copper oxides are ceramics with _
A) photovoltaic
Q116: If a face-centered cubic unit cell
Q118: The alpha form of polonium (Po) has
Q119: In a simple cubic cell there is/are
Q128: Gold (Au) has a face-centered cubic structure
Q185: When Ge is doped with Ga, it
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents