Consider a solution made by mixing 500.0 mL of 4.0 M NH3 and 500.0 mL of 0.40 M AgNO3. Ag+ reacts with NH3 to form AgNH3+ and Ag(NH3) 2+: 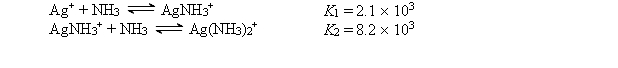 The concentration of Ag(NH3) 2+ at equilibrium is
The concentration of Ag(NH3) 2+ at equilibrium is
A) 0.20 M.
B) 2.0 M.
C) 0.40 M.
D) 1.0 * 10-3 M.
E) none of these
Correct Answer:
Verified
Q126: Determine the pH of a solution prepared
Q127: If 30 mL of 5.0 *10-4 M
Q128: Consider the titration of 200.0 mL of
Q129: The concentration of Mg2+ in seawater is
Q130: Calculate the minimum concentration of 100.0 mL
Q132: The observed solubility of the salt MX
Q133: How many moles of Ca(NO3)2 must be
Q134: A 50.0-mL sample of 2.0 *10-4 M
Q135: The Ksp for Mn(OH)2 is 2.0 *
Q136: A 200-mL solution contains 0.018 mol each
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents