Consider a solution made by mixing 500.0 mL of 4.0 M NH3 and 500.0 mL of 0.40 M AgNO3. Ag+ reacts with NH3 to form AgNH3+ and Ag(NH3) 2+: 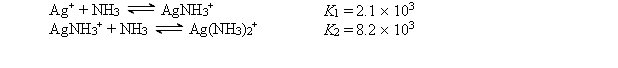 The concentration of Ag+ at equilibrium is
The concentration of Ag+ at equilibrium is
A) 2.0 M.
B) 4.5 * 10-9 M.
C) 1.2 *10-8 M.
D) 1.6 M.
E) none of these
Correct Answer:
Verified
Q142: You have 0.20 M HNO2 (Ka =
Q143: Explain how to decide on a specific
Q144: For the compound MX, Ksp is 2.00
Q148: Derive the equation describing the relationship between
Q149: Given the following values of equilibrium constants:
Q150: Explain why the pH of an aqueous
Q151: The cation M2+ reacts with NH3 to
Q152: Differentiate between the equivalence point and the
Q152: Calculate the pH of the final solution
Q160: Explain why we cannot directly compare Ksp
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents