Which metal, Al or Ni, could reduce Zn2+ to Zn(s) if placed in a Zn2+(aq) solution? 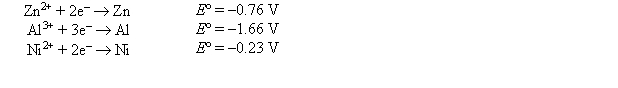
A) Ni
B) Al
C) Both Al and Ni would work.
D) Neither Al nor Ni would work.
E) This cannot be determined.
Correct Answer:
Verified
Q8: The following questions refer to a
Q9: Ammonium metavanadate reacts with sulfur dioxide
Q9: Silver will spontaneously reduce which of the
Q10: The following reaction occurs in basic
Q11: Determine the standard potential, E°, of
Q12: When the equation for the following
Q14: Which of the following is the strongest
Q17: What is the oxidation state of Mn
Q18: How many electrons are transferred in
Q19: Which of the following would be the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents