The standard free energies of formation of several aqueous species are 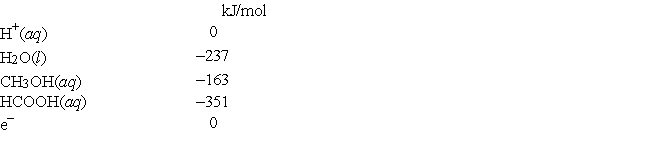 What is the standard reduction potential of methanoic acid in aqueous solution (that is, for HCOOH + 4H+ + 4e- → CH3OH + H2O) ?
What is the standard reduction potential of methanoic acid in aqueous solution (that is, for HCOOH + 4H+ + 4e- → CH3OH + H2O) ?
A) +0.13 V
B) +0.25 V
C) -0.25 V
D) -0.13 V
E) +0.17 V
Correct Answer:
Verified
Q27: Refer to the galvanic cell below (the
Q28: Choose the correct statement(s) given the following
Q29: The reaction Cr(s) + NO3-(aq) → Cr3+(aq)
Q30: Consider an electrochemical cell with a copper
Q31: Consider the galvanic cell shown below (the
Q33: Consider the hydrogen-oxygen fuel cell where
H2(g) +
Q34: Consider the following reduction potentials: 
Q35: Consider the galvanic cell shown below (the
Q36: Refer to the galvanic cell below (the
Q37: Refer to the galvanic cell below (the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents