Consider the galvanic cell shown below (the contents of each half-cell are written beneath each compartment) . 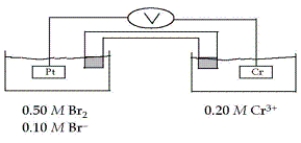 The standard reduction potentials are as follows:
The standard reduction potentials are as follows: 
What is the value of E for this cell at 25°C?
A) 1.88 V
B) 2.12 V
C) 1.76 V
D) 2.21 V
E) 0.59 V
Correct Answer:
Verified
Q45: A fuel cell designed to react grain
Q46: Refer to the galvanic cell below (the
Q47: For a reaction in a voltaic cell,
Q48: Consider an electrochemical cell that has a
Q49: If a reducing agent M reacts with
Q51: An excess of finely divided iron is
Q52: A cell is set up with copper
Q53: Consider an electrochemical cell with a copper
Q54: Refer to the galvanic cell below (the
Q55: You make a cell with a copper
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents