During a reversible adiabatic expansion of an ideal gas, which of the following is NOT true?
A) 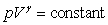
B) pV = nRT
C) 
D) W = - pdV
E) pV = constant
Correct Answer:
Verified
Q24: The energy absorbed as heat by an
Q34: The pressure of an ideal gas is
Q73: The heat capacity at constant volume of
Q100: The specific heat at constant volume of
Q102: When work W is done on an
Q103: TV
Q106: During a slow adiabatic expansion of a
Q107: The temperature of n moles of
Q109: When work W is done on an
Q110: Monatomic, diatomic, and polyatomic ideal gases each
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents