What is the net ionic equation for the reaction of any strong acid with a soluble,strong hydroxide base,such as NaOH?
A) H+(aq) + Na+(aq) H2O( 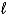 ) + Na(s)
) + Na(s)
B) H2(g) + 2 OH-(aq) 2 H2O( 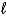 )
)
C) 2 H+(aq) + O(g) H2O(aq)
D) H+(aq) + OH-(aq) H2O( 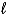 )
)
E) 3 H+(aq) + OH-(aq) H2(g) + H2O( 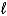 )
)
Correct Answer:
Verified
Q37: Write the net ionic equation for the
Q39: What is the net ionic equation
Q39: Write the net ionic equation for the
Q40: Ethane,C2H6 ,is a component of natural gas
Q41: Which of the following combinations of reactant
Q42: What appears on the right side of
Q45: In the balanced net ionic equation for
Q45: What is the preferred way to write
Q47: Which of the following could be a
Q49: Which of the following is a product
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents