Consider the following particulate-level representation of a reaction. 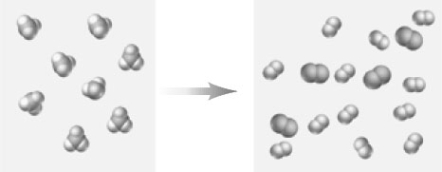 How would this reaction be classified.
How would this reaction be classified.
A) combination
B) decomposition
C) single-replacement
D) double-replacement
Correct Answer:
Verified
Q23: A precipitate forms when solutions of magnesium
Q24: Pure hydrogen iodide decomposes spontaneously to its
Q25: Examine the following two beakers. 
Q27: Molten sodium chloride is decomposed into its
Q29: Calcium nitrate and potassium fluoride solutions react
Q32: A precipitate forms when magnesium chloride and
Q32: Consider the following particulate-level representation of a
Q33: Calcium reacts with hydrobromic acid.Write the balanced
Q36: A piece of potassium is placed in
Q42: Sulfuric acid neutralizes a barium hydroxide solution.Write
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents