Faraday's Law of Electrolysis states that the mass of a substance produced at an electrode during electrolysis (m) is directly proportional to the number of moles of electrons transferred at that electrode (n) .Which of the following equations correctly characterizes the relation?
A) m = k*n
B) As the number of electrons transferred increases the mass deposited decreases.
C) There is one conversion factor possible that relates m and n.
D) 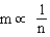
E) All of the above correctly describe this relation.
Correct Answer:
Verified
Q11: What will be the cost in dollars
Q16: Convert 3.77 *108 pL to mL,daL,and dL.1
Q18: A 60-kg astronaut travels from the earth
Q19: Complete the following operation: 9.370 *10-5 -
Q20: How many fluid ounces are in a
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents