If 10.0 g of aluminum is consumed in the reaction 2 NaOH(aq) + 2 Al(s) + 6 H2O( 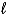 ) 2 NaAl(OH) 4(aq) + 3 H2(g) ,what volume of hydrogen,measured at -4°C and 0.88 atm,will be produced?
) 2 NaAl(OH) 4(aq) + 3 H2(g) ,what volume of hydrogen,measured at -4°C and 0.88 atm,will be produced?
A) 6.2 L
B) 12 L
C) 14 L
D) 16 L
E) 19 L
Correct Answer:
Verified
Q2: An unknown substance is vaporized at 200°C
Q18: Calculate the density of neon at 32°C
Q19: Calculate the density of nitrogen dioxide at
Q23: A gas has a density of 1.19
Q24: How many moles are in 1.73 L
Q24: How many grams of hydrogen chloride
Q25: If the molar volume of a gas
Q26: Calculate the molar volume of nitrogen at
Q29: What is the molar mass of a
Q33: What volume of oxygen,measured at STP,can be
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents