Which of these ions has a Lewis symbol of the following form? 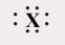
A) N3-
B) F+
C) C4+
D) Ar
E) O22-
Correct Answer:
Verified
Q5: Which of the following statements correctly describe
Q8: How is the bond in an F2
Q10: When a covalent bond forms,the atomic orbitals
Q11: Why does the stability of a noble
Q12: The general term to indicate a negatively
Q15: The ratio of anions to cations in
Q16: Which of the following ions is/are isoelectronic
Q19: Which of the following correctly describes a
Q20: Which of the following correctly describes a
Q26: Given the following electronegativities: C = 2.5,N
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents