Hydrogen peroxide decomposes to form water and oxygen gas according to the following equation: 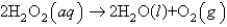 Suppose 145.9 g of hydrogen peroxide decomposes and all of the oxygen gas is collected in a balloon at 1.00 atm and 25oC. Determine the volume of the balloon.
Suppose 145.9 g of hydrogen peroxide decomposes and all of the oxygen gas is collected in a balloon at 1.00 atm and 25oC. Determine the volume of the balloon.
A) 4.40 L
B) 104.9 L
C) 52.4 L
D) 8.80 L
E) none of these
Correct Answer:
Verified
Q67: A compressed gas cylinder, at 137 atm
Q68: When acetylene gas, C2H2, burns, how many
Q69: A sample of argon gas occupies a
Q70: A sample of oxygen gas (O2) has
Q71: Solid carbon dioxide is placed into a
Q73: The largest a party balloon can get
Q74: You carry out the reaction represented
Q75: There are two balloons, both the same
Q76: Solid carbon dioxide is placed into a
Q77: A flexible weather balloon contains helium gas
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents