How many joules of energy would be required to heat 15.9 g of carbon from 23.6°C to 54.2°C? (Specific heat capacity of carbon = 0.71 J/g·°C.)
A) 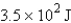
B) 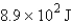
C) 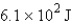
D) 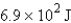
E) none of these
Correct Answer:
Verified
Q7: The specific heat capacity of iron is
Q8: Calculate the heat given off when 159.7
Q9: Assume that 491.8 J of heat is
Q10: Which is larger, one calorie or one
Q11: A system absorbs 159 kJ of heat,
Q13: How much energy will be needed to
Q14: The specific heat capacity of silver is
Q15: SI units for specific heat capacity are
A)
Q16: The quantity of heat required to change
Q17: A 6.75-g sample of gold (specific heat
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents