According to the first law of thermodynamics, the energy of the universe is constant. Does this mean that 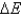 is always equal to zero?
is always equal to zero?
A) Yes, 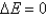 at all times, which is why
at all times, which is why 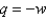 .
.
B) No, 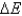 does not always equal zero, but this is due only to factors such as friction and heat.
does not always equal zero, but this is due only to factors such as friction and heat.
C) No, 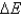 does not always equal zero because it refers to the system's internal energy, which is affected by heat and work.
does not always equal zero because it refers to the system's internal energy, which is affected by heat and work.
D) No, 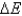 never equals zero because work is always being done on the system or by the system.
never equals zero because work is always being done on the system or by the system.
E) No, 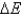 never equals zero because energy is always flowing between the system and surroundings.
never equals zero because energy is always flowing between the system and surroundings.
Correct Answer:
Verified
Q43: The first law of _ states that
Q44: On a cold winter day, a steel
Q45: Consider the following processes: Q46: Consider the reaction: Q47: _ was formed from the remains of Q49: The greenhouse effect occurs only on the Q50: _ is a measure of disorder or Q51: Consider the following standard heats of formation: Q52: Consider the following reaction: Q53: In the equation Unlock this Answer For Free Now! View this answer and more for free by performing one of the following actions Scan the QR code to install the App and get 2 free unlocks Unlock quizzes for free by uploading documents![]()
![]()
![]()
![]()

