The balanced equation 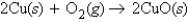 tells us that 4.0 mol of Cu
tells us that 4.0 mol of Cu
A) reacts with 4.0 mol of O2
B) produces 4.0 mol of CuO
C) must react with 128 g of O2
D) cannot react with oxygen
E) produces 8.0 mol of CuO
Correct Answer:
Verified
Q8: The rusting of iron is represented by
Q9: The equation Q10: Refer to the following unbalanced equation: Q11: Refer to the following equation: Q12: A 3.1-mol sample of KClO3 was decomposed Q14: An excess of Al and 7.9 mol Q15: A mole ratio is used to convert Q16: How many molecules of carbon dioxide will Q17: A balanced chemical equation is one that Q18: Which of the following reaction mixtures would![]()

Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents