Consider the equation: 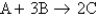 . The molar mass of B is 50.0 g/mol. Which of the following statements is true when equal masses of A and B are reacted?
. The molar mass of B is 50.0 g/mol. Which of the following statements is true when equal masses of A and B are reacted?
A) If the molar mass of A is greater than the molar mass of B, then A must determine how much C is produced.
B) If the molar mass of A is less than the molar mass of B, then A must determine how much C is produced.
C) If the molar mass of A is the same as the molar mass of B, then A and B react in a perfect stoichiometric ratio and both determine how much C is produced.
D) If the molar mass of A is greater than the molar mass of B, then B must determine how much C is produced.
E) If the molar mass of A is less than the molar mass of B, then B must determine how much C is produced.
Correct Answer:
Verified
Q60: Fe2O3 (molar mass = 159.7 g/mol) reacts
Q61: Consider a reaction in which two reactants
Q62: Consider the equation: A + 4B
Q63: Consider the following unbalanced equation: 
Q64: Consider the following reaction at 1.10 atm
Q66: Consider the following unbalanced equation: 
Q67: Which of the following statements is always
Q68: For the titration of sulfuric acid (H2SO4)
Q69: When 10.0 mol of calcium metal is
Q70: Consider that calcium metal reacts with oxygen
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents