An experiment requires 66.6 g of ethyl alcohol (density = 0.790 g/mL) . What volume, in liters, will be required?
A) 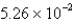 L
L
B) 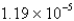 L
L
C) 8.43e4 L
D) 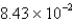 L
L
E) 52.6 L
Correct Answer:
Verified
Q87: At 20°C the density of mercury is
Q88: The Celsius equivalent of 62.2 K is
A)
Q89: Convert: 18.6°F = _ °C.
A) 28.11°C
B) -24.12°C
C)
Q90: Cesium melts at 302 K and boils
Q91: An experiment requires 74.2 mL of ethyl
Q93: If a 100.-g sample of platinum metal
Q94: Water has a density of 1.0 g/mL.
Q95: Density is an example of a
A) chemical
Q96: If a 100.-g sample of a metal
Q97: Convert: -12.2°C = _ °F.
A) -54.0°F
B) 10.0°F
C)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents